About Ferric Carboxymaltose 50 mg/mL IVCROS FCM
Composition:
IVCROS FCM is an intravenous iron preparation containing Ferric Carboxymaltose (50 mg elemental iron per mL) as the active ingredient. It is a stable, non-dextran iron complex designed for rapid iron replenishment in patients with iron deficiency.
Indications:
IVCROS FCM is indicated for the treatment of iron deficiency anemia (IDA) in adult and pediatric patients (as per approved age group) when oral iron therapy is ineffective or cannot be tolerated. It is also used in conditions where rapid iron correction is required, such as chronic kidney disease (CKD), inflammatory bowel disease (IBD), or postpartum anemia.
Dosage & Administration:
-
Administered intravenously by slow injection or infusion under medical supervision.
-
Dosage is individualized based on the patient s body weight and total iron deficit.
-
Maximum single dose: Up to 1000 mg (20 mL) in adults, as per clinical guidelines.
Advantages:
-
Rapid and complete iron correction in 1-2 infusions.
-
Minimal risk of anaphylaxis (non-dextran formulation).
-
Well-tolerated with a low incidence of hypersensitivity reactions.
Side Effects:
Common adverse reactions may include nausea, headache, dizziness, flushing, or injection site reactions. Serious hypersensitivity reactions, though rare, require immediate medical attention.
Contraindications:
-
Hypersensitivity to ferric carboxymaltose or any excipients.
-
Non-iron deficiency anemia (e.g., hemochromatosis).
Manufacturer: AKUMS Drugs & Pharmaceuticals Ltd.
Marketed By: Neon Laboratories Ltd.
Disclaimer:
This product should be used under strict medical supervision. The information provided here is for educational purposes only and does not replace professional medical advice. Always consult a qualified healthcare provider for diagnosis, treatment, and dosage recommendations. The manufacturer does not guarantee the accuracy or completeness of this information and disclaims liability for any adverse effects arising from its use.
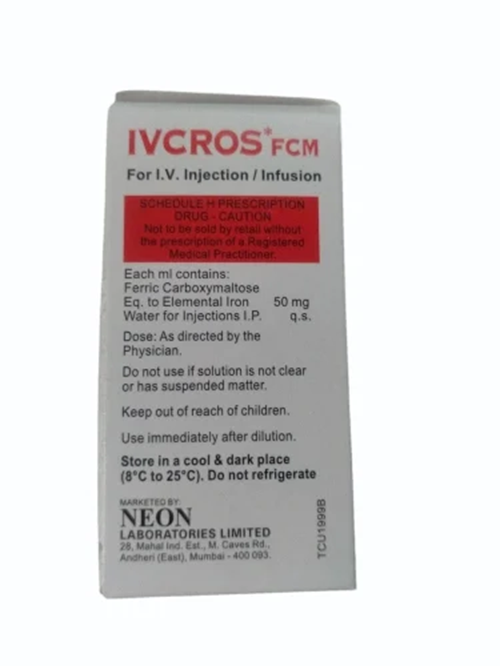
Storage: Store below 30C, protect from light. Keep out of reach of children.
Effective Management of Iron Deficiency AnemiaFerric Carboxymaltose IVCROS FCM is indicated for rapid recovery in patients suffering from iron deficiency anemia. Its high assay value (99%) ensures potent and consistent therapeutic response. By replenishing iron stores efficiently, this intravenous formulation supports hematological health, catering to a diverse patient population requiring timely intervention.
Quality, Safety, and Ease of UseProduced under stringent pharmaceutical standards, IVCROS FCM is supplied as a sterile, aqueous solution with a convenient single-use vial package. The product's stability, sterility, and precise dosage make it a reliable choice in clinical settings, contributing to patient safety and streamlined administration.
FAQ's of Ferric Carboxymaltose 50 mg/mL IVCROS FCM:
Q: How is Ferric Carboxymaltose 50 mg/mL IVCROS FCM administered?
A: Ferric Carboxymaltose IVCROS FCM is administered intravenously by healthcare professionals. The solution is delivered directly into a vein, ensuring rapid absorption and optimal efficacy for treating iron deficiency anemia.
Q: What conditions does this product treat?
A: This intravenous iron therapy is indicated for the treatment of iron deficiency anemia, particularly in patients who cannot tolerate oral iron supplements or need quick replenishment of iron stores.
Q: When should Ferric Carboxymaltose IVCROS FCM be used?
A: This medication should be used when iron deficiency anemia has been diagnosed by a physician and oral iron treatments are either ineffective or not tolerated. It is intended for clinical administration under medical supervision.
Q: Where is the product manufactured and supplied from?
A: Ferric Carboxymaltose 50 mg/mL IVCROS FCM is manufactured and exported from India. It is available through authorized pharmaceutical exporters, suppliers, traders, and wholesalers.
Q: What is the process for ordering and storing this medication?
A: The minimum order quantity is 10 vials, with each box containing 10 ml sterile vials. Upon receipt, the vials should be stored below 25C and protected from light to maintain product potency and sterility.
Q: How is this injection beneficial compared to oral iron preparations?
A: Ferric Carboxymaltose IV injection provides rapid and reliable replenishment of body iron stores, circumventing gastrointestinal side effects often associated with oral iron, and is suitable for patients requiring fast correction of anemia.
Q: What are the key handling and expiration details for Ferric Carboxymaltose FCM?
A: This medicine must be stored in cool, light-protected conditions and used within 24 months from the manufacturing date. Always check the expiration date before use and ensure the solution remains sterile and free from contamination.